GMO- Genetically modified organisms (Plants and Animals) have been around since the 1990s, first approval for human consumption was given in 1994 in the USA. In crop farming and livestock breeding, Genomes have been modified in laboratories to favour particular traits in the end organism. Selective breeding has been around for even longer. In GMOs, selected genes from unrelated species have been altered at the molecular level to get desired traits. However, GMO technology has been rather imprecise, with a large degree of randomness and a kind of brute force approach where changes have to be made gene by gene taking up immense time with unpredictable results.
CRISPR technology has changed that.
Jennifer Doudna and Emmanuelle Charpentier won the 2020 Nobel Prize in Chemistry. This was based on their joint research and publication of a paper in Science in 2012. They discovered how CRISPR-Cas systems, are adaptive immune response systems that protect bacteria from viruses by cleaving their nucleic acids using a protein Cas9.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is akin to a gene editing tool that can be used to modify the DNA of organisms. It works by using a protein called Cas9, which acts like a pair of molecular scissors, to cut the DNA at a specific location.
The process of using CRISPR for gene editing typically involves designing a guide RNA (gRNA) that is complementary to a specific sequence of the DNA that is to be edited. The gRNA is then combined with the Cas9 protein and introduced into the cells of the organism.
Once inside the cell, the Cas9 protein and gRNA form a complex that can recognize and bind to the target DNA sequence. The Cas9 protein then cuts the DNA at the specific location, creating a double-strand break in the DNA.
The cell’s natural DNA repair mechanisms try to repair the break, but the process can be manipulated to introduce specific changes in the DNA sequence. For example, researchers can provide a new DNA template that contains the desired changes, and the cell’s repair machinery will use that template to repair the break, incorporating the desired changes into the DNA.
This process can be used to create specific changes in the DNA sequence, such as inserting, deleting, or replacing specific genes.
The gRNA molecule acts as a sort of “address label” that directs the Cas9 protein to the specific location in the DNA where the cut should be made.
CRISPR promises to revolutionize health care, agriculture, livestock, poultry, fisheries, and virtually any domain in the Life Sciences realm. It sounds good; but when will financial results flow?

The low-hanging fruit is in Agriculture, Livestock, Poultry, and Fisheries. Presently about 800 million people in the world are undernourished, and 2 Billion are micro nutrient deficient. As per estimates, the world will need to feed 10 Billion people by 2050. That is a 56% calorie gap. This food gap needs a huge increase in crop productivity and nutrition. Present land under agriculture shall have to increase by a land area two times the size of India. Nitrogen-based fertilizer usage with environmental issues will need to increase and lastly, GHG emissions shall have to reduce to 4GT CO2 from about 40 GT presently to contain global temperatures below 2 Degree centigrade. These changes are not happening this is a crisis!
Using CRISPR it is possible to create plants with higher crop yield, disease and drought resistance, and higher nutrition. Crops like wheat and rice consume immense amounts of Nitrogen fertilizer this is partially consumed and leaches into the ground, water bodies, and oceans creating algal blooms and environmental hazards. Using CRISPR, a legume’s Nitrogen-fixing gene can be introduced in other plant species reducing the need for artificial fertilizer. Companies such as DuPont, Monsanto (now owned by Bayer), and Syngenta are using CRISPR. The present global Gross value of agricultural products as per FAO is US$ 5 Trillion.
Humans consume about 350 million tonnes of meat products. With increasing income, the per capita demand shall rise. In Animal Sciences-livestock, poultry and fish with more nutrition, disease, pest resistance and other traits can be precisely engineered. Companies such as Intelllia, Editas, Recombnetics, Horizon and the Roslin Institute are working in this field.
In biotechnology and pharmaceuticals, CRISPR is being used to develop new treatments for genetic diseases, cancer, and other conditions. Companies such as CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics, and Beam Therapeutics are using CRISPR to develop new gene therapies and drug discovery platforms.
The real breakthrough for CRISPR in disease cure came in the research of Sickle-cell disease.
Sickle cell is caused by inheriting two bad copies of one of the genes that make hemoglobin. Symptoms include bouts of intense pain, and life expectancy with the disease is just 53 years. It affects 1 in 4,000 people in the US, nearly all of them African-American.
So how did this disease become CRISPR’s first success? Our bodies harbor another way to make hemoglobin that turns off when we’re born. Researchers found that a simple DNA edit to cells from the bone marrow could turn it back on.
In 2022, Vertex Pharmaceuticals, based in Boston, was the first company to bring Sickle-cell therapy to regulators for approval. The treatment regimen is complex. It involves a hospital stay; doctors remove the bone marrow, edit the cells, and then transplant them back. To cure it, Vertex and its partner company, CRISPR Therapeutics, aren’t fixing the genes responsible for the mutation that leaves those molecules misshapen. Instead, the new treatment involves a kind of molecular bank shot—an edit that turns on fetal hemoglobin, a second form of the molecule that we have in the womb but loses as adults.
Some of a patient’s stem cells are removed from the blood with a filtering machine, and the CRISPR cutting protein is added to them with an electrical jolt so that it can seek out and break into the BCL11A gene, the one that controls the production of fetal hemoglobin. The edited cells are then dripped back into a blood vessel. They multiply and start making fetal hemoglobin.
But the expected price tag of the gene-editing treatment is $2 to $3 million. Since the disease is prevalent in Africa, there is no way this treatment will be implemented there. This is more like an experiment and will ultimately be used as a cure for many other diseases. So simpler, cheaper ways to deliver CRISPR will have to come next. With R&D, regulatory approvals, clinical trials, etc, we are easily looking at 5-10 years before CRISPR goes mainstream in humans.
On the other hand, the Vertex treatment is a landmark because we are now in the era of commercial rewriting of human genomes. “It’s a huge milestone in the history of humankind and an important stepping-stone to what will be possible in the future,” says William Pao, a former head of drug development at Pfizer.
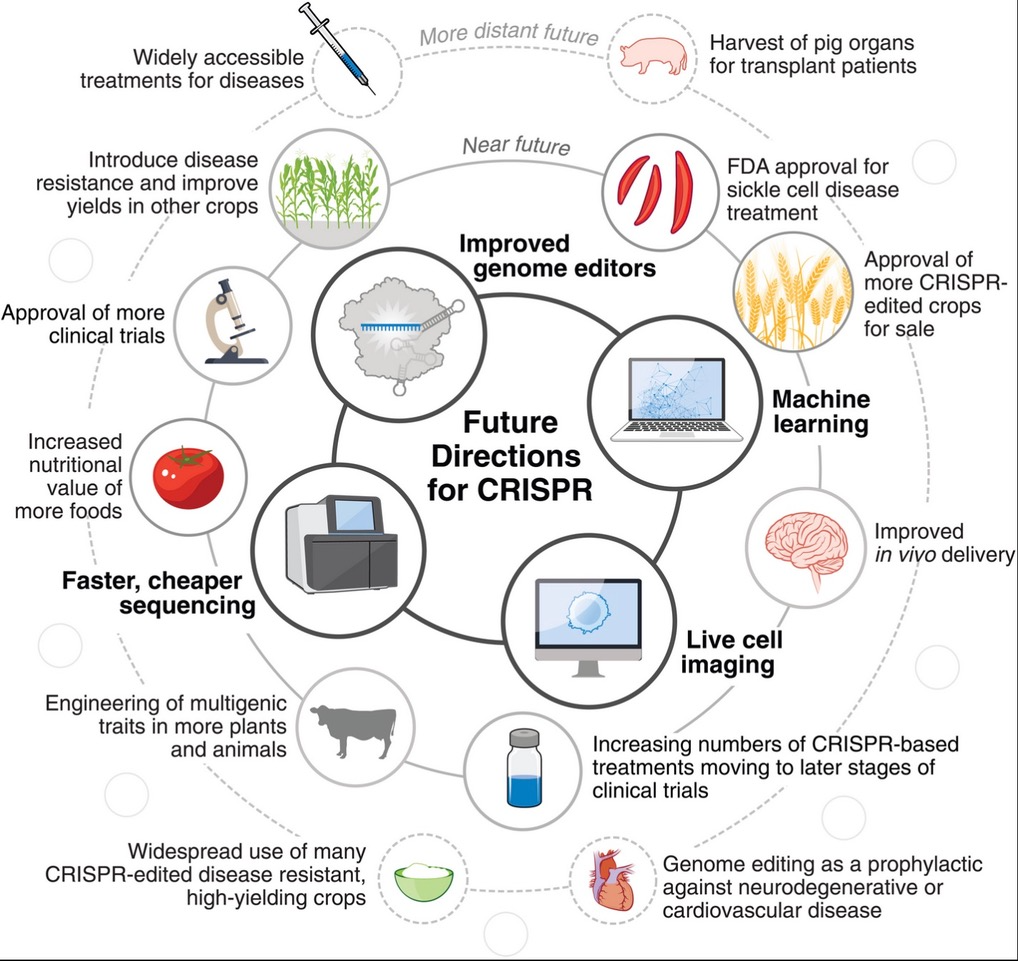
Now here is the paradox! We have a revolutionary technology under development for the last decade, it is a milestone in gene editing and promises to solve many global problems; however, investments, trials, and regulatory approvals are time-consuming. The ownership of intellectual property rights to the technology is foggy. The Broad Institute of MIT and Harvard were awarded several patents while the University of California, Berkeley, and University of Vienna, were where Doudna and Charpentier conducted much of their early research.
Lastly, there is a stigma on the term “Gene Editing”.
A lot of things will need to happen for CRISPR to deliver and create commercially successful products and cures.
In contrast, AI and LLM’s have attracted over US$ 50 Billion in investments in about a year! I don’t think their impact on mankind’s problems shall be as impactful, however, their ability to succeed is extremely high. The reallocation of investments and missionary zeal in bringing CRISPR-like technologies is the need of the day.
Molecular biology, genetics, and genomics stand at a pivotal moment in history, where the convergence of knowledge, computing, imaging, and techniques has empowered us to edit specific base pairs or segments of DNA within cells and living organisms. This opens a vast ocean of opportunity! Will it translate into money?
Acknowledgement: I convey my thanks for the images and material from “CRISPR technology: A decade of genome editing is only the beginning” by Joy Y. Wang and Jennifer A. Doudna* published in Science 379 on 20th Jan 2023
Leave a Reply